Stable Electroporation-Competent Cell說明書
產品貨號: ML-G27007
保存條件: -80℃
產品規格: 5×50μl 20×50μl
產品介紹
基 因 型
F' proA+B+ lacIq ?(lacZ)M15 zzf::Tn10 (TetR) ?(ara-leu) 7697 araD139 fhuA ?lacX74 galK16 galE15e14- Φ80dlacZ?M15 recA1 relA1 endA1 nupG rpsL (StrR) rph spoT1 ?(mrr-hsdRMS -mcrBC)
簡 要 說 明
Stable 電擊感受態細胞只能用于電擊轉化,不可用于熱激轉化。-MLBio
Stable 菌株是NEB 公司開發的高轉化效率菌株,是逆轉錄病毒/慢病毒載體系統推薦使用的菌株,具有與Stbl2, Stbl3完全不同的基因型,但是表現出比Stbl2,Stbl3 更優異的性能,特別適合慢病毒或具有末端重復序列DNA 片段的克隆。
基因組含有重組酶recA1 rel A1突變,可有效抑制長片段末端重復區的重組,降低錯誤重組的概率;同時含有核酸酶 endA1突變,避免了提取質粒過程中核酸酶的污染,大大提高了高純度病毒質粒的產量和質量。lacZΔM15 的存在使 Stable 可用于藍、白斑篩選,此菌株具有四環素和鏈霉素抗性.
MLBio開發的Stable電擊感受態細胞適用于大質粒的構建或者各種具有末端重復序列的復雜 DNA文庫構建,經特殊工藝制作,pUC19質粒檢測轉化效率>2×1010 cfu/μg DNA。
操 作 說 明
1.0.1cm 電擊杯和杯蓋從儲存液中拿出倒置于干凈的吸水紙上 5分鐘,待其瀝干水分,正置 5分鐘,使乙醇充分揮發,待乙醇揮發干凈立即插入冰中,壓實冰面,電極杯頂離冰面 0.5 cm以方便蓋上杯蓋,冰中靜置 5分鐘充分降溫。
2.取-80℃保存的Stable電擊感受態細胞插入冰中5 分鐘,待其融化,加入目的 DNA (質粒或連接產物)并用手撥打EP 管底輕輕混勻,避免產生氣泡,立即插入冰中。
A. 測定轉化效率使用 1 μl 10 pg/μl 的對照質粒 pUC19;
B. 對于連接產物,請用乙醇沉淀DNA 后加入適量 TE緩沖液 (10mM TrisHCl, pH7.5;1mM EDTA)重懸,DNA 濃度不超過100ng/μl,體積不超過 5μl/50μl 感受態
3.用200 μl槍頭將感受態-DNA 混合物快速移到電擊杯中,避免產生氣泡,蓋上杯蓋。
4.啟動電轉儀,設置參數:C=25 μF,PC=200 Ω,V=1.8 kV (此為 BioRad 電轉儀推薦參數,也可按所用 電轉儀推薦的參數操作),將電擊杯快速放入電轉槽中,電擊完成快速插入冰中。
5.立即 向電擊杯中加入1000 μl不含抗生素的無菌培養基 S.O.C.,混勻后轉移到空50ml管中,并用1ml SOC輕柔沖洗電轉杯并轉移到50ml管中,另補加3ml SOC培養基, 37℃,200 rpm復蘇60分鐘。
6.5000 rpm 離心一分鐘收菌,重懸后取 100-200 μl 涂布到含相應抗生素的 S.O.C 平板上(因菌量較大, 若全部涂板請選用直徑 15cm 培養皿 2-5 個)。將平板倒置放于 37℃培養箱過夜培養過夜。
注 意 事 項
1.加入DNA 時體積不應大于感受態體積的1/10。
2.電擊感受態細胞加入電擊杯應避免產生氣泡,氣泡會增加弧光放電風險。
3.當DNA 不純或存在鹽,乙醇,蛋白及緩沖液等污染時,轉化效率急劇下降。
4.電擊杯里的離子可增加溶液的電導,增大在含有細胞和DNA 的溶液中產生電流和弧光放電的風險。
5.若轉化大質粒或想獲得較高轉化效率,推薦使用高純質粒提取試劑盒提取質粒。質粒增大一倍,轉化效率下降一個數量級。
6.對于連接產物轉化, 最好轉化前乙醇沉淀DNA后用適量TE緩沖液 (10 mMTrisHCl,pH7.5;1 mMEDTA)重懸產物,保證 DNA 濃度不超過 100ng/μl。過高濃度連接產物或過大體積連接產物會降低轉化效率,增加弧光放電的風險。
7.混入質粒時應輕柔操作,吸取感受態細胞時不可用孔徑過小的槍頭 (普通 200ul 槍頭應剪去槍頭尖0.6cm)避免用力過猛,以免剪切力過大損傷細胞膜,降低轉化效率。轉化高濃度的質粒或連接產物可相應減少最終用于涂板的菌量。
8.電擊感受態細胞最好保存在-80℃以下,高于-80℃超期儲存會導致轉化效率會下降。
酶聯生物經過不斷的實驗優化和改進,積累了大量的經驗,擁有專業的酶聯研發團隊。利用專業的酶聯免疫技術自主研發的elisa試劑盒,能對血清及其它樣本定量檢測抗原,定性檢測特異性抗體。優質的試劑,先進的儀器和正確的操作是保證ELISA檢測結果準確可靠的必要條件。ELISA檢測的方便性、穩定性、重復性和可靠性方面都具有很大的優勢。
ELISA檢測技術服務內容:
1、雙抗體夾心法檢測抗原 2、間接法檢測抗體 3、為客戶提供各種ELISA技術進行樣本檢測。
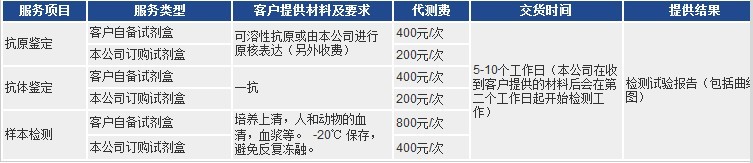
以上代測費,凡購買本公司試劑盒,我們免費代測!
凡購買本公司目錄任何一種酶聯免疫檢測試劑盒,您只需將需要檢測的動物(Human, Rat, Mouse, Rabbit, Monkey,
Pig……)種類和檢測指標(白介素類、激素類)及標本數量(48T/96T)通知公司業務員即可。在接到客戶標本當日起,現貨產品一周內將檢測報告交到客戶手中!
歡迎各科研單位在各種項目上與我們公司開展不同層次的密切合作,以雙贏求發展,共同進步,為中國檢測事業的發展積累經驗。
二、樣本要求
在收集標本前都必須有一個完整的計劃,必須清楚要檢測的成份是否足夠穩定。我們提倡新鮮標本盡早檢測,對收集后當天就進行檢測的標本,及時儲存在4℃備用,如有特殊原因需要周期收集標本,請造模取材后,將標本及時分裝后放在-20℃或-70℃條件下保存。因冰室與室溫存在一定溫差,蛋白極易降解,直接影響實驗質量,所以避免反復凍融。代測放免標本的客戶取材前須向我司銷售人員索要說明書,具體操作注意事項請與我司技術人員溝通。
液體類標本:標本必須為液體,不含沉淀。包括血清、血漿、尿液、胸腹水、腦脊液、細胞培養上清、組織勻漿等。
血清:室溫血液自然凝固10-20分鐘后,離心20分鐘左右(2000-3000轉/分)。收集上清。如有沉淀形成,應再次離心。
血漿:應根據試劑盒的要求選擇EDTA、檸檬酸鈉或肝素作為抗凝劑,加入10%(v/v)抗凝劑(0.1M檸檬酸鈉或1%heparin
或2.0%EDTA.Na2)混合10-20分鐘后,離心20分鐘左右(2000-3000轉/分)。仔細收集上清。如有沉淀形成,應再次離心。
尿液、胸腹水、腦脊液:用無菌管收集。離心20分鐘左右(2000-3000轉/分)。仔細收集上清。如有沉淀形成,應再次離心。
細胞培養上清:檢測分泌性的成份時,用無菌管收集。離心20分鐘左右(2000-3000轉/分)。仔細收集上清。檢測細胞內的成份時,用PBS(PH7.0-7.4)稀釋細胞懸液,細胞濃度達到100萬/ml左右。通過反復凍融,以使細胞破壞并放出細胞內成份。離心20分鐘左右(2000-3000轉/分)。仔細收集上清。保存過程中如有沉淀形成,應再次離心。
組織標本:切割標本后,稱取重量。加入一定量的PBS,緩沖液中可加入1μg/L蛋白酶抑制劑或50U/ml的Aprotinin(抑肽酶)。用手工或勻漿器將標本勻漿充分。離心20分鐘左右(2000-3000轉/分)。仔細收集上清置于-20度或-70度保存,如有必要,可以將樣品濃縮干燥。分裝后一份待檢測,其余冷凍備用。
三、寄標本時需注明以下情況:
1、標本編號;2、所測項目;3、是否做復孔;3、聯系方式;4、實驗后標本是否寄回。
客戶須知:
客戶應對所提供的材料及信息負責,如因客戶提供的材料及信息不準確而引起的實驗延誤或經濟損失由客戶承擔。
Q:1.
how to collect samples and preparation of ELISA?
Performed by ELISA test is generally common clinical samples including blood (finger
blood, blood), urine, feces, cerebrospinal fluid, pleural effusion, prostatic fluid,
semen, vaginal secretions, which
Some time of sample collection, preservation methods and has certain requirements.
Collection (a) clinical specimens
A, blood samples:Some physiological factors, such as smoking, eating, exercise, mood
swings, pregnancy, postural changes in blood can affect certain ingredients, even some
of diurnal variation. Therefore, blood samples
Acquisition should avoid interference physiological factors, consistent with appropriate
conditions, such as can not be avoided, should indicate the factors on the specimen.
1. Peripheral:Usually select the inside of blood left ring finger, the portion should be
no frostbite, inflammation, edema, damage. If the site does not meet the requirements to
other parts of the fingers instead. For burn patients, optional leather
Intact skin at the blood. As part of routine blood tests (eg, white blood cell count,
sort, etc.) affected by physiological factors fluctuation is too large, when compared to
the conditional should be consistent. It relates to the body, blood clotting function
Can test items (such as platelet count, bleeding time or clotting time) testing, we must
pay attention to understand whether the patient used anticoagulant, procoagulant drugs
in order to reduce or avoid interfering factors
influences.
2. Blood:In addition to involving a variety of projects such as hemostasis and
thrombosis detector requires the use of anticoagulated blood plasma, the current
analysis to detect the vast majority of projects can be directly detected using blood
serum. In the serum test items
, Some (such as blood sugar, blood fat) diet and circadian factors influenced, fasting
blood samples were generally appropriate; some decay rapidly in the blood (serum enzyme
activity assay such as ACP activity, etc.),
0 ~ 4 ℃ storage is not an activity decreased, the detection of these projects must be
timely and fast; some (such as creatine kinase) influenced by exercise and other
factors. Avoid hemolysis occurs when blood is also important
And, more particularly potassium, LDH and other measurement.
B, urine samples:With the same blood samples, urine samples affect diet, exercise,
medication and other factors that are also large, especially on the diet, so the morning
urine generally superior to random urine. Means getting up early morning urine
After the first urine specimens, representing concentrated and acidified visible
components (such as blood cells, epithelial cells, tubular) easy to observe the relative
concentration. Random urine that is a random urine specimens convenient, but by diet,
Sports, and even more the influence of drugs, prone to false positive and false negative
results, such as diet proteinuria, glucosuria diet, vitamin C interference occult blood
results and the like. Postprandial urine (patient 2 hours after lunch, collected
Human Urine) suitable for urine, urine protein and urobilinogen check urine samples at
this time to increase the sensitivity of the test, the detection of minor lesions. 12
hours in urine cell count is Addis count (last night 8:00
After emptying the bladder to all specimens of urine 8 o'clock the next morning),
because a long time, easy to breed bacteria shall be added preservative formaldehyde.
24-hour urine (the first day of the morning after emptying the bladder specimens from
8:00 to 8:00 the next morning
All urine) quantification of chemical substances, including proteins, sugars, urinary
17-one, 17-hydroxy steroids, catecholamines, Ca2 +, etc., to detect different
substances, choose a different preservative preservative. clean
Urine used for urine bacterial culture requires sterile specimens were taken after
washing the vulva. Urine specimens should be enough to collect all, at least 12 ml,
preferably 50 ml, the timing must collect all the urine of women
Patients should avoid vaginal secretions, blood contamination of urine specimens.
C, stool samples:Stool samples for the detection judgment digestive diseases has
important reference value. Collection requirements with a clean bamboo select faecal
mucus, pus and blood components and other abnormality, no abnormal appearance
Droppings shall be drawn from multiple surface and deep manure end. Get parasitemia and
for egg counts should be collected 24 hours feces. Dysentery amoeba trophozoites check
should immediately check in after a bowel movement, and from there sepsis
Softer at the drawn, insulation inspection. Charles S. japonicum eggs should take mucus,
pus and blood portion 30g stool specimens from at least miracidia hatching, and to be
treated as soon as possible. Check pinworm eggs must use transparent film swab
Night before 12:00 or early in the morning from defecation wrinkled folds around the
anus and immediately swabbing at microscopic examination. Occult blood test (chemistry),
fasting before the test on the 3rd of meat and foods containing animal blood and ban
clothing iron, vitamin C and so on.
Should be checked in all 1 hour stool specimen collection is completed, in order to
prevent damage to physical components of digestive enzymes and pH by. For clinical
samples above the detection indicators.
D, CSF samples:CSF samples collected immediately after submission, place too long will
affect the test results: such as cell degeneration, destruction, leading to counting and
classification are not allowed; some chemicals such as glucose content will decompose
Save
Less; bacteria occur autolysis affect bacteria detection rate. Cerebrospinal fluid
extracted three general dispensing a sterile tube, the first tube for bacterial culture,
a second tube for chemical analysis and immunological tests, the third tube for general
Characters and microscopic examination, three of the order should be reversed. Specimen
collection is difficult because all inspection and testing process should pay attention
to safety.
E, ascites and pleural effusion samples:CSF samples with the same attention to safety
after the specimen collection, and timely submission. Generally separated into three
tubes, one for routine cytology, a biochemical examination, a bacterial culture, in
order
CSF same is appropriate.
F, prostatic fluid sample:Prostatic fluid specimen after prostate massage by the
acquisition, directly drop when less liquid on a glass slide and timely submission shall
be taken to prevent sample evaporation to dryness, the amount collected for a long time
in a clean, dry test tube. If massage
No prostatic fluid, urine sediment can be checked after the massage.
G, semen samples:Abstinence before semen collection should be 3 to 7 days, drain the
urine after masturbation or other available methods of semen directly into clean
containers, insulation and timely submission. Due to changes in sperm production during
the day and
Large, generally should be checked 2 to 3 times (each time interval of 1 to 2 weeks) in
order to make a diagnosis.
H, samples of vaginal secretions:Vaginal samples were collected 24 hours before
intercourse should be prohibited, bath, vaginal examination, vaginal lavage and local on
the drug, etc., drawing instruments used need to be cleaned. Usually with brine-soaked
cotton swab from the vagina deep
Or rear vaginal fornix, cervical canal mouth drawn, etc., made after saline smear
vaginal secretion samples observation, women with menstrual vaginal secretions were not
checking.
2, do before each sample by ELISA experiment how to prepare?
Before collecting the sample must have a comprehensive plan must clearly be detected
component is stable enough. To be collected on the same day
Sample testing, and timely backup stored at 4 ℃. For the next day re-testing samples
frozen in a timely manner after dispensing -20 ℃ spare, conditional, preferably -70 ℃
cryopreservation standby. Avoid repeated freezing and thawing specimens
.
Liquid samples: including serum, plasma, urine, pleural effusion, cerebrospinal fluid,
cell culture supernatant and the like.
1. serum:
Coagulation at room temperature 10-20 mins, centrifugation 20 minutes or so (2000-3000
rev / min). Carefully collect the supernatant. If precipitation during storage,
Centrifugal again.
2. Plasma:
EDTA should be selected according to the requirements of the specimen, sodium citrate or
heparin as an anticoagulant, mix 10-20 mins, centrifugation 20 minutes or so (2000-3000
rev / min). Carefully collect the supernatant. Save process
If precipitation appeared, Centrifugal again.
3. Urine:
Sterile collection tube. Centrifuged for 20 minutes or so (2000-3000 rev / min).
Carefully collect the supernatant. If precipitation during storage, Centrifugal again.
Pleural and peritoneal effusions, and cerebrospinal fluid Reference to this practice.
4. The cell culture supernatant:
The detection of secretory component with a sterile collection tube. Centrifuged for 20
minutes or so (2000-3000 rev / min). Carefully collect the supernatant.
5. cultured cells
????When the detection of intracellular components, diluted with PBS (PH7.2-7.4) cell
suspension, the cell concentration reached 1 million / ml or so. By repeated freezing
and thawing or tissue protein extraction reagent was added to the cells
Damage and release of intracellular components. Centrifuged for 20 minutes or so
(2000-3000 rev / min). Carefully collect the supernatant. If precipitation during
storage, Centrifugal again.
6. tissues
????After cutting samples, check the weight. Adding a certain amount of PBS, PH7.4.
Rapidly frozen with liquid nitrogen. After thawing samples remained at 2-8 ℃. Adding a
certain amount of PBS
(PH7.4), or tissue protein extraction reagent, or by hand homogenizer homogenized
sample. Centrifuged for 20 minutes or so (2000-3000 rev / min). Carefully collect the
supernatant. A new package to be detected, which
I alternate freezing.
Q:Do
I have to run all of my standards and samples in duplicate?
A:Yes, the duplicates are run in order to monitor assay precision and increase
confidence in the assay results obtained.
Q:Do
I have to run all of my samples at one time?
A:No, each kit uses stripwell microplate. This allows the user to analyse different
numbers of samples at different times.
Q:What
types of reproducible results are obtained with the assays?
A:Each kit comes with a manual containing a graph of typical data obtained. Any
variation in operator, pipetting and washing technique, incubation time or temperature,
and kit age can cause variation in result. Each user should obtain their own standard
curve.
Q:Is
it possible to store the reagents other than indicated?
A:Storage of the kit components under conditions other than indicated is not recommended
in order to assure proper performance of the test.
Q:How
should I store my samples?
A:Samples should be stored at -20oC or lower temperature. For long-term storage, it is
recommended to freeze them at -70oC -80oC.
Q:Can
I modify the protocol?
A:BG ELISA kits have been optimized to provide the best possible results. Modifying the
format or protocol may give inaccurate and wrong results.
Q:Can
I use a sample type that is not recommended in the kit insert?
A:The kit has been validated for the sample types listed in the kit insert. Sample types
other than those validated have not been tested. Contact Technical Service for further
information.
Q:My
samples generated values that were outside the dynamic range of the assay. Can I use
these values?
A:It is recommended that only sample values that fall within the range of the standard
curve be used. Values outside the range of the standard curve are generally non-linear,
which can lead to incorrectly extrapolated values. Samples that generate values higher
than the highest standard should be (further) diluted and the assay repeated. If samples
fall below the range of the assay, the sample is considered to be non-detectable.
Q:Do
I have to run a Blank or Zero Standards every time?
A:Yes, these are required for the calculations, and reflect any subtle but significant
performance changes from day to day and assay to assay. They are also extremely helpful
when troubleshooting the source of a particular assay problem.
Q:Can
I alter the volume of sample I use in the assay?
A:It is not recommended that you alter the volumes since all BG kits are designed for
optimal performance at the given volumes
Q:Can
components from different kits be used?
A:Each kit contains components which have specific lot numbers to ensure that all of the
components are performing optimally alone, as well as with all of the other components
in the kit. QC testing is performed on these specific lots. It is never recommended to
use your own components or components from other kits or vendors.
Q:My
standard curve looked fine, but I didn’t get a signal in my sample when I expected
to, why?
A:The sample may not contain the analyte. A matrix effect may be masking the detection.
Ensure that the recommended dilution was followed as stated in the kit insert. If
dilution was recommended, check to be sure that the dilution was performed properly.
Over-dilution may cause the sample to fall below the range of the standard curve.
Q:How
do you recommend I wash my plate?
A:If you are using an automated plate washer we recommend that the calibration be
checked on a regular basis, and that the system is flushed with the Plate Washing Buffer
prior to washing. The same is true for a manual washer. A repeater or a wash bottle can
also be used. The user should be careful to ensure that all of the contents are
aspirated and the plate tapped dry on lint-free paper.
Q:Do
I need to use a plate shaker?
A:Reliable results can be obtained without a plate shaker, but the O.D.'s will generally
be lower than those obtained using a plate shaker.
Q:Why
do I have to use wavelength correction between 450-570nm?
A:For the ELISA assay, reading at dual wavelengths is done to correct for the optical
density contributed by the plastic well, the lamp and optical fluctuations.
Q:If
I extract my sample, do I still need to follow the recommended dilutions given in
the kit insert?
A:The amount of sample dilution needed after an extraction procedure will be affected by
the effects of purification and concentration in the protocol used. The amount of
dilution or concentration will have to be determined by the end-user.
Q:What
is the expected concentration of analyte that I should expect to find?
A:The amount of a given analyte may vary not only from species-to-species, but also
between tissue and cellular sources. The best source of this information is the current
literature that is easily accessed through the Internet at multiple scientific
databases.
Q:My
optical densities were a little higher (or lower) than those in the manual that came
with my kit. Why?
A:The optical density is affected by a number of physical conditions such as time and
temperature. We suggest that you shorten or lengthen the final incubation with substrate
solution to compensate.
Q:What
are the reasons for High Background?
A:1) Improper Washing: Check volume of washing buffer reservoir and make sure all
recommended washing steps are performed.
2) Contaminated Substrate: Make sure there is no contamination of the substrate with
metal ions or oxidizing reagents, before use. Keep the extra substrate solution
separately during the ELISA substrate development time.
3) Substrate exposed to light: Exposure to light may result in a blue color of the
substrate. Keep solutions in the dark (vial) until ready to dispense into the plate.
4) Wrong Incubation Times/Temperatures: Generally follow the test protocol regarding
incubation times and temperatures. However, if all wells are intensely and equally
colored with no intensity gradient observed in the standard dilution series, then it may
be necessary to observe the substrate reaction as the color is developing, in order to
stop the reaction sooner.


 酶聯官方手機二維碼
酶聯官方手機二維碼







































